- Diabetes, Obesity and Metabolism
- EW-7197 Attenuates the Progression of Diabetic Nephropathy in db/db Mice through Suppression of Fibrogenesis and Inflammation
-
Kyung Bong Ha, Weerapon Sangartit, Ah Reum Jeong, Eun Soo Lee, Hong Min Kim, Soyeon Shim, Upa Kukongviriyapan, Dae-Kee Kim, Eun Young Lee, Choon Hee Chung
-
Endocrinol Metab. 2022;37(1):96-111. Published online February 28, 2022
-
DOI: https://doi.org/10.3803/EnM.2021.1305
-
-
3,996
View
-
180
Download
-
3
Web of Science
-
3
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
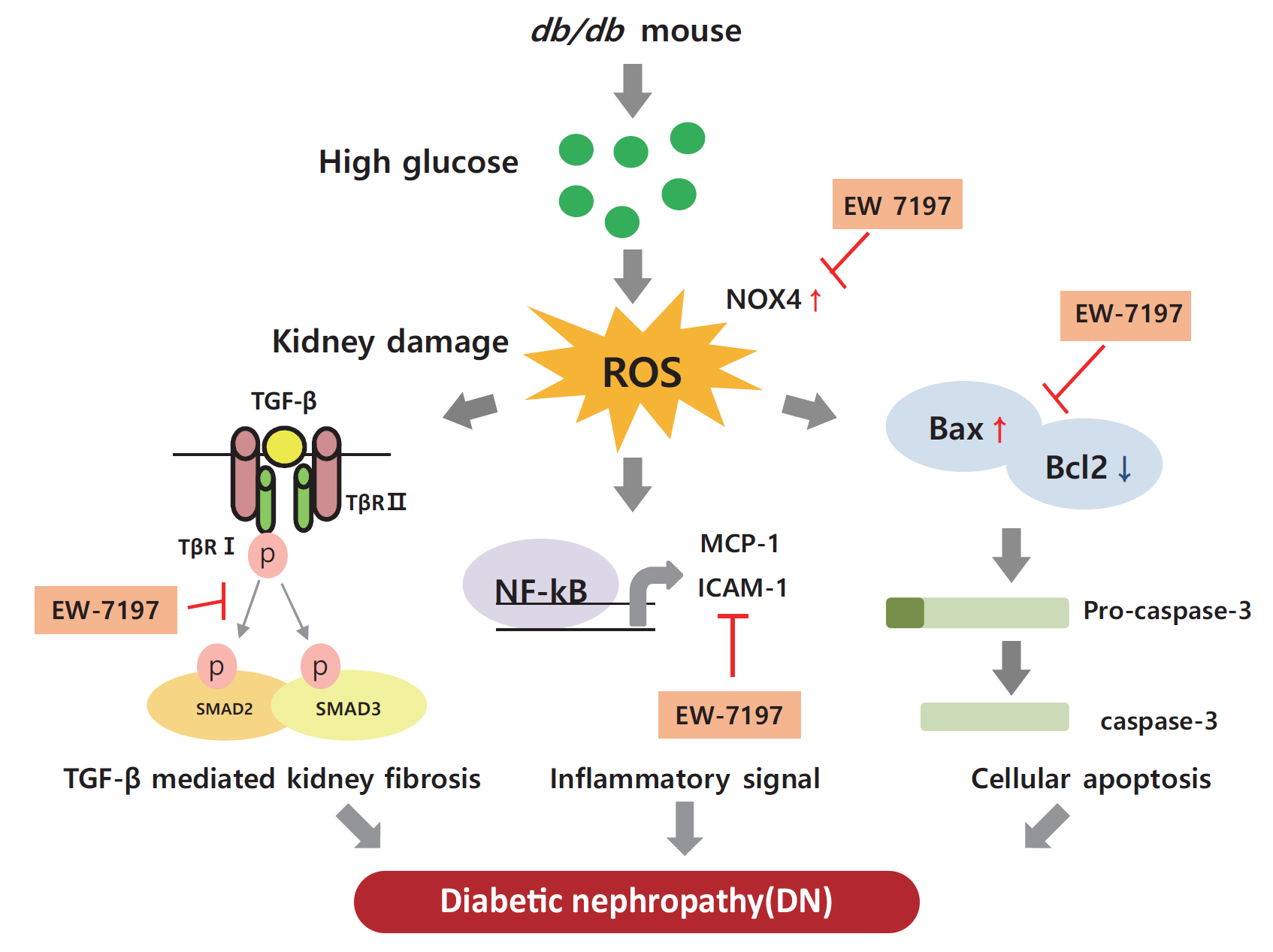
Diabetic nephropathy (DN) is characterized by albuminuria and accumulation of extracellular matrix (ECM) in kidney. Transforming growth factor-β (TGF-β) plays a central role in promoting ECM accumulation. We aimed to examine the effects of EW-7197, an inhibitor of TGF-β type 1 receptor kinase (ALK5), in retarding the progression of DN, both in vivo, using a diabetic mouse model (db/db mice), and in vitro, in podocytes and mesangial cells.
Methods
In vivo study: 8-week-old db/db mice were orally administered EW-7197 at a dose of 5 or 20 mg/kg/day for 10 weeks. Metabolic parameters and renal function were monitored. Glomerular histomorphology and renal protein expression were evaluated by histochemical staining and Western blot analyses, respectively. In vitro study: DN was induced by high glucose (30 mM) in podocytes and TGF-β (2 ng/mL) in mesangial cells. Cells were treated with EW-7197 (500 nM) for 24 hours and the mechanism associated with the attenuation of DN was investigated.
Results
Enhanced albuminuria and glomerular morphohistological changes were observed in db/db compared to that of the nondiabetic (db/m) mice. These alterations were associated with the activation of the TGF-β signaling pathway. Treatment with EW-7197 significantly inhibited TGF-β signaling, inflammation, apoptosis, reactive oxygen species, and endoplasmic reticulum stress in diabetic mice and renal cells.
Conclusion
EW-7197 exhibits renoprotective effect in DN. EW-7197 alleviates renal fibrosis and inflammation in diabetes by inhibiting downstream TGF-β signaling, thereby retarding the progression of DN. Our study supports EW-7197 as a therapeutically beneficial compound to treat DN.
-
Citations
Citations to this article as recorded by  - TGF-β signaling in health, disease, and therapeutics
Ziqin Deng, Tao Fan, Chu Xiao, He Tian, Yujia Zheng, Chunxiang Li, Jie He
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols
Qi Jin, Tongtong Liu, Yuan Qiao, Donghai Liu, Liping Yang, Huimin Mao, Fang Ma, Yuyang Wang, Liang Peng, Yongli Zhan
Frontiers in Immunology.2023;[Epub] CrossRef - Beneficial Effects of a Curcumin Derivative and Transforming Growth Factor-β Receptor I Inhibitor Combination on Nonalcoholic Steatohepatitis
Kyung Bong Ha, Eun Soo Lee, Na Won Park, Su Ho Jo, Soyeon Shim, Dae-Kee Kim, Chan Mug Ahn, Choon Hee Chung
Diabetes & Metabolism Journal.2023; 47(4): 500. CrossRef
- Diabetes, Obesity and Metabolism
- Tetrahydrocurcumin Ameliorates Kidney Injury and High Systolic Blood Pressure in High-Fat Diet-Induced Type 2 Diabetic Mice
-
Weerapon Sangartit, Kyung Bong Ha, Eun Soo Lee, Hong Min Kim, Upa Kukongviriyapan, Eun Young Lee, Choon Hee Chung
-
Endocrinol Metab. 2021;36(4):810-822. Published online August 27, 2021
-
DOI: https://doi.org/10.3803/EnM.2021.988
-
-
4,059
View
-
164
Download
-
7
Web of Science
-
5
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
Activation of the intrarenal renin-angiotensin system (RAS) is implicated in the pathogenesis of kidney injury and hypertension. We aimed to investigate the protective effect of tetrahydrocurcumin (THU) on intrarenal RAS expression, kidney injury, and systolic blood pressure (SBP) in high-fat diet (HFD)-induced type 2 diabetic mice.
Methods
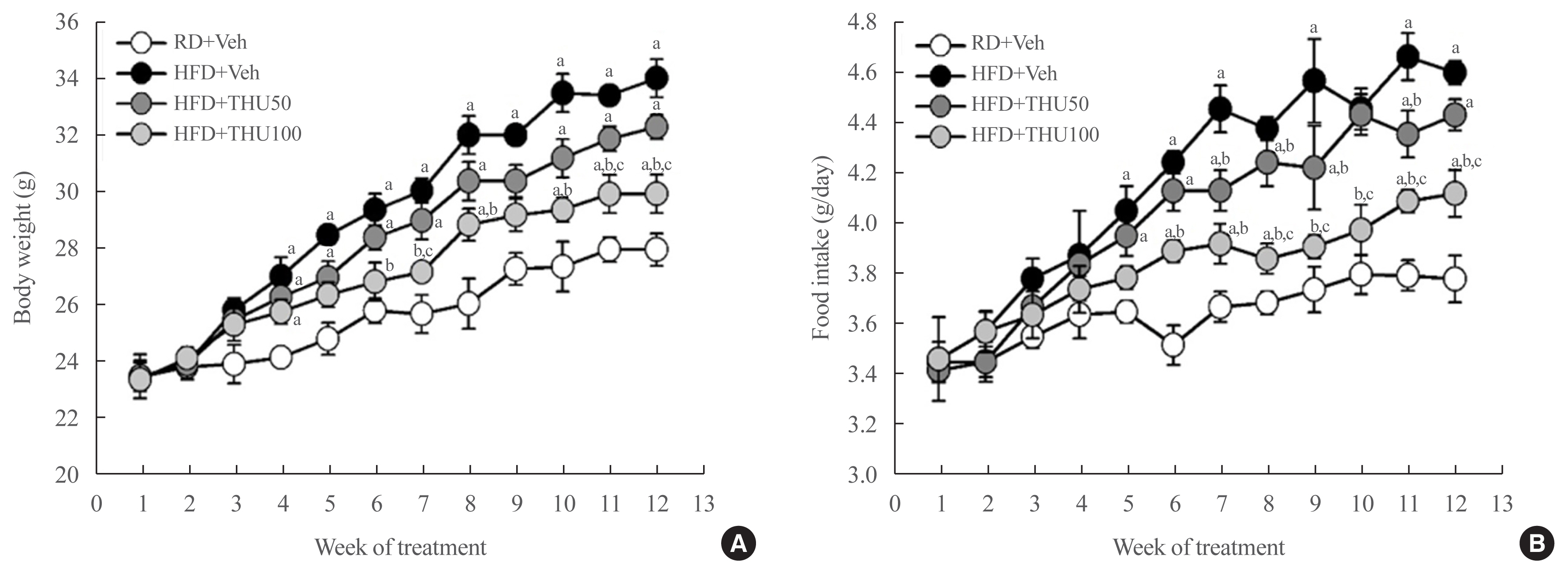
Eight-week-old male mice were fed a regular diet (RD) or HFD for 12 weeks, and THU (50 or 100 mg/kg/day) was intragastrically administered with HFD. Physiological and metabolic changes were monitored and the expression of RAS components and markers of kidney injury were assessed.
Results
HFD-fed mice exhibited hyperglycemia, insulin resistance, and dyslipidemia compared to those in the RD group (P<0.05). Kidney injury in these mice was indicated by an increase in the ratio of albumin to creatinine, glomerular hypertrophy, and the effacement of podocyte foot processes. Expression of intrarenal angiotensin-converting enzyme, angiotensin II type I receptor, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-4, and monocyte chemoattractant protein-1 was also markedly increased in HFD-fed mice. HFD-fed mice exhibited elevated SBP that was accompanied by an increase in the wall thickness and vascular cross-sectional area (P<0.05), 12 weeks post-HFD consumption. Treatment with THU (100 mg/kg/day) suppressed intrarenal RAS activation, improved insulin sensitivity, and reduced SBP, thus, attenuating kidney injury in these mice.
Conclusion
THU alleviated kidney injury in mice with HFD-induced type 2 diabetes, possibly by blunting the activation of the intrarenal RAS/nicotinamide adenine dinucleotide phosphate oxidase IV (NOX4)/monocyte chemoattractant protein 1 (MCP-1) axis and by lowering the high SBP.
-
Citations
Citations to this article as recorded by  - The Development of Dyslipidemia in Chronic Kidney Disease and Associated Cardiovascular Damage, and the Protective Effects of Curcuminoids
Zeltzin Alejandra Ceja-Galicia, Ana Karina Aranda-Rivera, Isabel Amador-Martínez, Omar Emiliano Aparicio-Trejo, Edilia Tapia, Joyce Trujillo, Victoria Ramírez, José Pedraza-Chaverri
Foods.2023; 12(5): 921. CrossRef - Translation Animal Models of Diabetic Kidney Disease: Biochemical and Histological Phenotypes, Advantages and Limitations
Wenting Luo, Shiyun Tang, Xiang Xiao, Simin Luo, Zixuan Yang, Wei Huang, Songqi Tang
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 1297. CrossRef - Curcumin ameliorates focal segmental glomerulosclerosis by inhibiting apoptosis and oxidative stress in podocytes
Hui Zhang, Qing-Qing Dong, Hua-Pan Shu, Yu-Chi Tu, Qian-Qian Liao, Li-Jun Yao
Archives of Biochemistry and Biophysics.2023; 746: 109728. CrossRef - An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy
Xiaoyu Zhu, Xingli Xu, Chigang Du, Yanping Su, Lixue Yin, Xiaoqiu Tan, Hui Liu, Yiru Wang, Lei Xu, Xinghua Xu
Biomedicine & Pharmacotherapy.2022; 153: 113438. CrossRef - An integrated bioinformatics analysis and experimental study identified key biomarkers CD300A or CXCL1, pathways and immune infiltration in diabetic nephropathy mice
WEI LIANG, QIANG LUO, ZONGWEI ZHANG, KEJU YANG, ANKANG YANG, QINGJIA CHI, HUAN HU
BIOCELL.2022; 46(8): 1989. CrossRef
- Endocrine Research
- Effects of Oxytocin on Cell Proliferation in a Corticotroph Adenoma Cell Line
-
Jung Soo Lim, Young Woo Eom, Eun Soo Lee, Hyeong Ju Kwon, Ja-Young Kwon, Junjeong Choi, Choon Hee Chung, Young Suk Jo, Eun Jig Lee
-
Endocrinol Metab. 2019;34(3):302-313. Published online September 26, 2019
-
DOI: https://doi.org/10.3803/EnM.2019.34.3.302
-
-
5,008
View
-
74
Download
-
3
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
Oxytocin (OXT) has been reported to act as a growth regulator in various tumor cells. However, there is a paucity of data on the influence of OXT on cell proliferation of corticotroph adenomas. This study aimed to examine whether OXT affects cell growth in pituitary tumor cell lines (AtT20 and GH3 cells) with a focus on corticotroph adenoma cells. MethodsReverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay were conducted with AtT20 cells to confirm the effects of OXT on hormonal activity; flow cytometry was used to assess changes in the cell cycle after OXT treatment. Moreover, the impact of OXT on proliferating cell nuclear antigen (PCNA), nuclear factor κB, and mitogen-activated protein kinase signaling pathway was analyzed by Western blot. ResultsOXT treatment of 50 nM changed the gene expression of OXT receptor and pro-opiomelanocortin within a short time. In addition, OXT significantly reduced adrenocorticotropic hormone secretion within 1 hour. S and G2/M populations of AtT20 cells treated with OXT for 24 hours were significantly decreased compared to the control. Furthermore, OXT treatment decreased the protein levels of PCNA and phosphorylated extracellular-signal-regulated kinase (P-ERK) in AtT20 cells. ConclusionAlthough the cytotoxic effect of OXT in AtT20 cells was not definite, OXT may blunt cell proliferation of corticotroph adenomas by altering the cell cycle or reducing PCNA and P-ERK levels. Further research is required to investigate the role of OXT as a potential therapeutic target in corticotroph adenomas.
-
Citations
Citations to this article as recorded by  - Increased proliferation and neuronal fate in prairie vole brain progenitor cells cultured in vitro: effects by social exposure and sexual dimorphism
Daniela Ávila-González, Italo Romero-Morales, Lizette Caro, Alejandro Martínez-Juárez, Larry J. Young, Francisco Camacho-Barrios, Omar Martínez-Alarcón, Analía E. Castro, Raúl G. Paredes, Néstor F. Díaz, Wendy Portillo
Biology of Sex Differences.2023;[Epub] CrossRef - Anterior pituitary gland synthesises dopamine from l‐3,4‐dihydroxyphenylalanine (l‐dopa)
Santiago Jordi Orrillo, Nataly de Dios, Antonela Sofía Asad, Fernanda De Fino, Mercedes Imsen, Ana Clara Romero, Sandra Zárate, Jimena Ferraris, Daniel Pisera
Journal of Neuroendocrinology.2020;[Epub] CrossRef
- Polarized and Stage-Dependent Distribution of Immunoreactivity for Novel PDZ-Binding Protein Preso1 in Adult Neurogenic Regions
-
Eun Soo Lee, Woon Ryoung Kim, Younghwa Kim, Hyun Woo Lee, Hyun Kim, Woong Sun
-
Endocrinol Metab. 2014;29(3):349-355. Published online September 25, 2014
-
DOI: https://doi.org/10.3803/EnM.2014.29.3.349
-
-
3,852
View
-
35
Download
-
2
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
- Background
Adult neural stem cells have the potential for self-renewal and differentiation into multiple cell lineages via symmetric or asymmetric cell division. Preso1 is a recently identified protein involved in the formation of dendritic spines and the promotion of axonal growth in developing neurons. Preso1 can also bind to cell polarity proteins, suggesting a potential role for Preso1 in asymmetric cell division. MethodsTo investigate the distribution of Preso1, we performed immunohistochemistry with adult mouse brain slice. Also, polarized distribution of Preso1 was assessed by immunocytochemistry in cultured neural stem cells. ResultsImmunoreactivity for Preso1 (Preso1-IR) was strong in the rostral migratory stream and subventricular zone, where proliferating transit-amplifying cells and neuroblasts are prevalent. In cultured neural stem cells, Preso1-IR was unequally distributed in the cell cytosol. We also observed the distribution of Preso1 in the subgranular zone of the hippocampal dentate gyrus, another neurogenic region in the adult brain. Interestingly, Preso1-IR was transiently observed in the nuclei of doublecortin-expressing neuroblasts immediately after asymmetric cell division. ConclusionOur study demonstrated that Preso1 is asymmetrically distributed in the cytosol and nuclei of neural stem/progenitor cells in the adult brain, and may play a significant role in cell differentiation via association with cell polarity machinery.
-
Citations
Citations to this article as recorded by  - FERM domain–containing proteins are active components of the cell nucleus
Péter Borkúti, Ildikó Kristó, Anikó Szabó, Zoltán Kovács, Péter Vilmos
Life Science Alliance.2024; 7(4): e202302489. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
|